Tolerability
Tolerability
▼ LYFNUA® (gefapixant) is non-narcotic with a safety profile established in two 52-week phase 3 clinical trials1,2
Adverse reactions in patients treated with LYFNUA® in two phase 3 clinical studies (cough-1 and cough-2):3
System Organ Class
Adverse Reactions
| Infections and infestations | |
| Common | Upper respiratory tract infection |
| Metabolism and nutrition disorders | |
| Common | Decreased appetite |
| Nervous system disorders | |
| Very Common | Dysgeusia,a Ageusia, Hypogeusia |
| Common | Taste disorder, Dizziness |
| Respiratory, thoracic and mediastinal disorders | |
| Common | Cough,b Oropharyngeal pain |
| Gastrointestinal disorders | |
| Common | Nausea, Diarrhea, Dry mouth, Salivary hypersecretion, Abdominal pain upper, Dyspepsia, Hypoesthesia oral, Paresthesia oral |
| Psychiatric disorders | |
| Common | Insomnia |
| Renal and urinary disorders | |
| Uncommon | Calculus urinary, Nephrolithiasis, Calculus bladder |
a Dysgeusia was commonly reported as taste bitter, taste metallic or taste salty.
b Cough includes reports of ‘worsening’, ‘exacerbation’, ‘increase’, or ‘increased’ cough.
Note: Ageusia was defined as a loss of taste. Hypogeusia was defined as diminished taste.2
The adverse reactions reported with LYFNUA® obtained from clinical studies are listed in the table above by MedDRA system organ class and by frequency. Frequencies are defined as very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1000 to <1/100), rare (≥1/10,000 to <1/1000), and very rare (<1/10,000).3

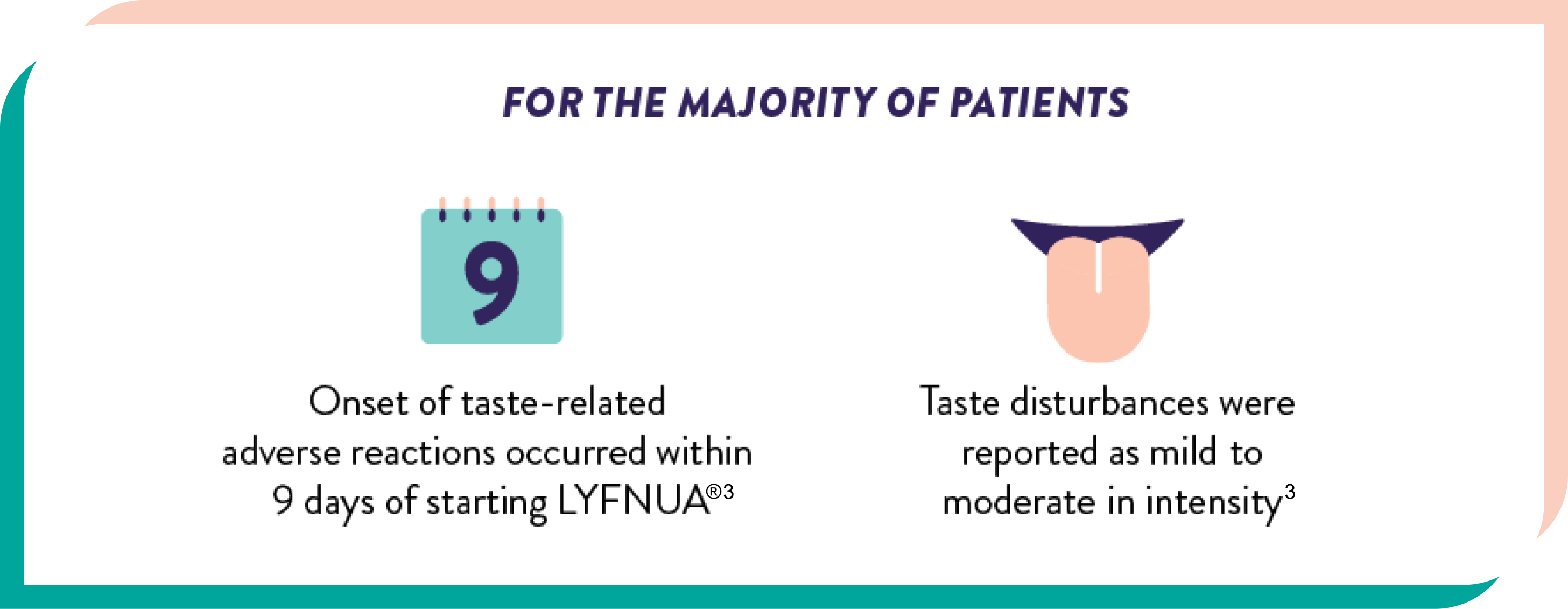
Adverse reactions resulting in discontinuation occurred in 22% of patients receiving LYFNUA®3

The most frequently reported adverse reactions leading to discontinuation of treatment were dysgeusia (9%) and ageusia (4%)3
INDICATION
LYFNUA® is indicated in adults for the treatment of refractory or unexplained chronic cough (RCC).3
References:
- Muccino D, Green S. Update on the clinical development of gefapixant, a P2X3 receptor antagonist for the treatment of refractory chronic cough. Pulm Pharmacol Ther. 2019 Jun;56:75-78.
- McGarvey LP, Birring SS, Morice AH et al., COUGH-1 and COUGH-2 Investigators. Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet. 2022 Mar 5;399(10328):909-923.
- LYFNUA®, Summary of product characteristics, 09/2023, section 4.1 and 4.8
▼ LYFNUA® (gefapiksant) 45 mg filmdrasjerte tabletter
Terapeutisk indikasjon: Lyfnua er indisert til voksne til behandling av refraktær eller uforklarlig kronisk hoste.
Dosering og administrasjonsmetode: Anbefalt dose av Lyfnua er én 45 mg tablett tatt oralt to ganger daglig med eller uten mat. Pasienter med alvorlig nyresvikt (eGFR < 30 mL/minutt/1,73 m²) som ikke krever dialyse, bør få en redusert dose på én 45 mg tablett som tas én gang daglig.
Pakker og priser (MRP): 56 filmdrasjerte tabletter (blisterpakke), 45 mg: kr 1445,90. Reseptgruppe: C.
Utvalgt sikkerhetsinformasjon:
Spesielle advarsler og forholdsregler for bruk: For pasienter med OSA (obstruktiv søvnapné), bør passende behandling for OSA vurderes før behandling med Lyfnua igangsettes. Lyfnua bør brukes med forsiktighet hos pasienter med kjent overfølsomhet for sulfonamider. Behandling med gefapiksant skal vurderes og tilpasses hos pasienter som utvikler en akutt infeksjon i nedre luftveier.
Fertilitets-, graviditets- og ammeinformasjon: Bruk av Lyfnua bør unngås under graviditet, hos kvinner med reproduksjonsmuligheter som ikke bruker prevensjon, og for ammende kvinner.
Bivirkninger: De vanligste bivirkningene med Lyfnua (som kan påvirke mer enn 1 av 10 personer) inkluderer smakforstyrrelser: dysgeusi (endringer i smakssansen, 41%), ageusi (tap av smakssans, 15%) og hypogeusi (redusert smakssans, 11%).
Konsulter LYFNUA SmPC 09/2023 før forskrivning eller bruk for fullstendig informasjon om dosering, kontraindikasjoner, advarsler og forholdsregler, interaksjoner og bivirkninger.
MSD (Norge) AS, Postboks 1S79 Vika, 0118 Oslo, tlf. 32 20 73 00 faks 32 20 73 10.
Copyright © 2025 Merck & Co., lnc., Rahway, NJ, USA og dets datterselskaper. Alle rettigheter forbeholdt.
NO-NON-00562