Efficacy
Efficacy
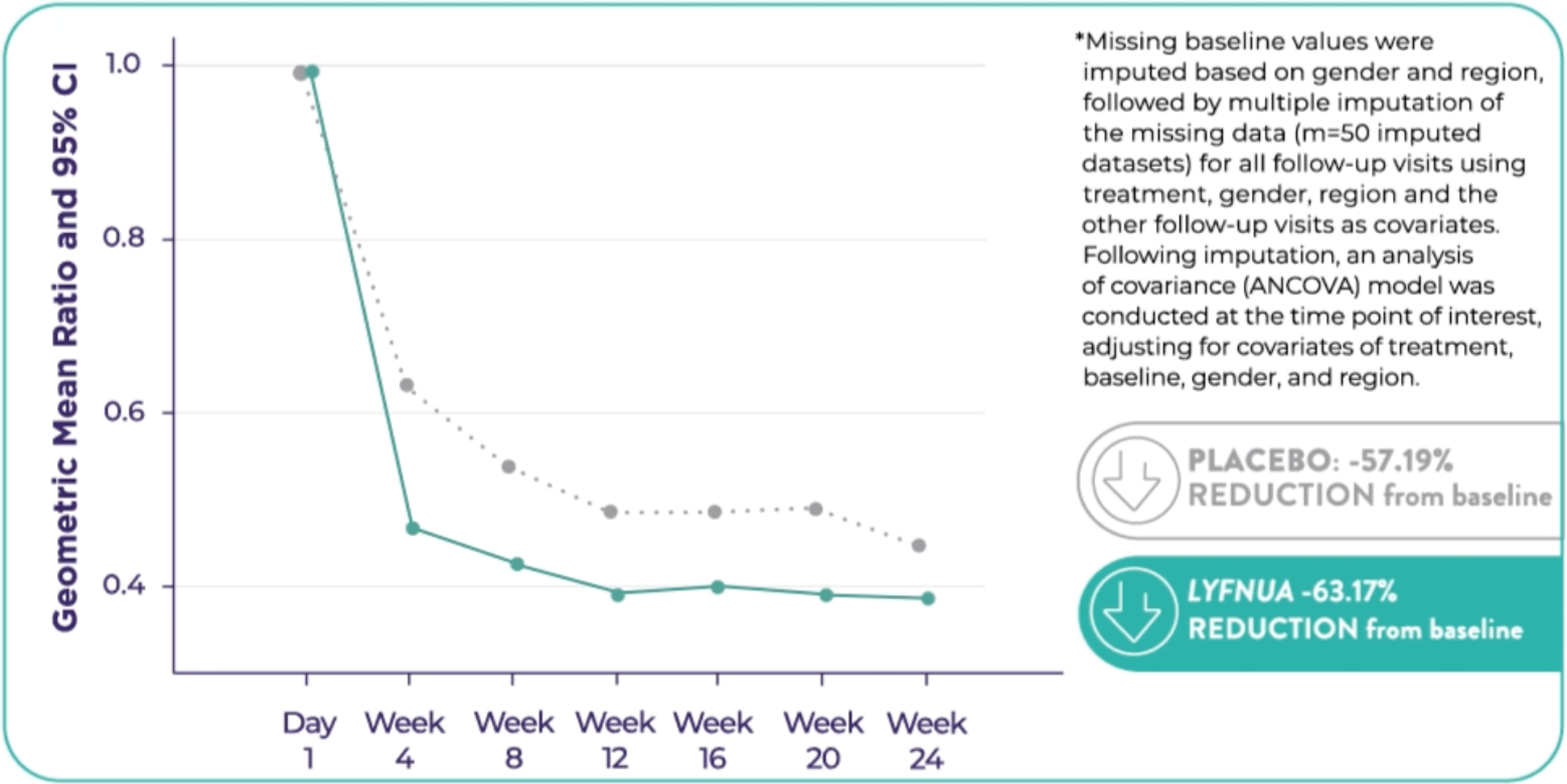
▼ LYFNUA® (gefapixant) is proven to help reduce 24-hour cough frequency and improve cough-specific quality of life1
Patients taking LYFNUA® 45 mg twice daily showed a statistically significant reduction in 24-hour cough frequency relative to placebo in two phase 3 clinical trials.1
The reduction in 24-hour cough frequency was observed by Week 4 and persisted throughout 12 and 24 weeks.1
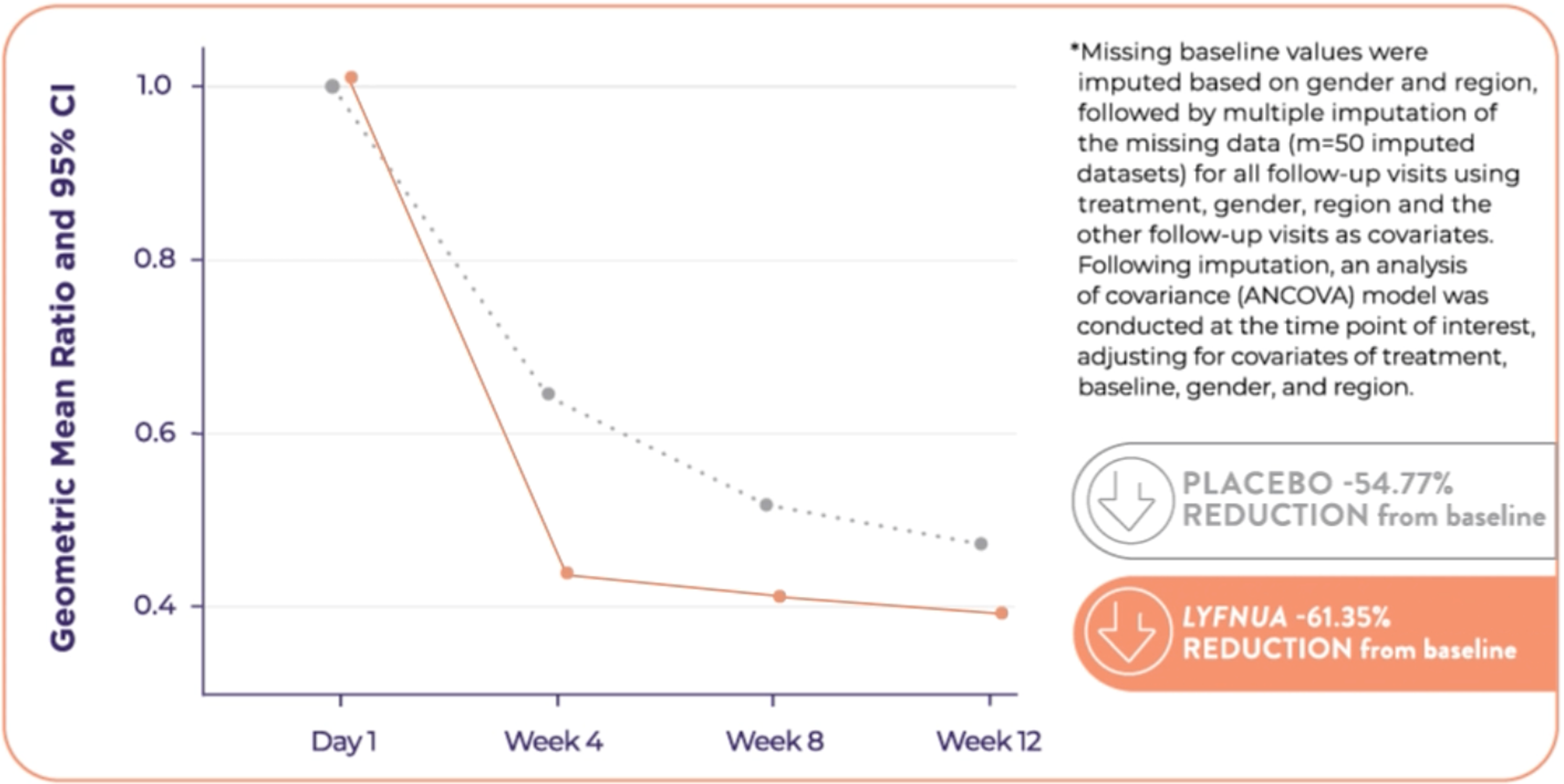
COUGH-1: Reduction in 24-hour cough frequency at 12 weeks relative to placebo was -18.52%* (95% CI: -32.76, -1.28; p=0.036)
*Based on a population pharmacokinetic analysis, age, body weight, gender, ethnicity, and race do not have a clinically meaningful effect on the pharmacokinetics of LYFNUA®.
Clinical Study Design
EXPLORE THE PHASE 3 CLINICAL TRIALS
A landmark clinical program
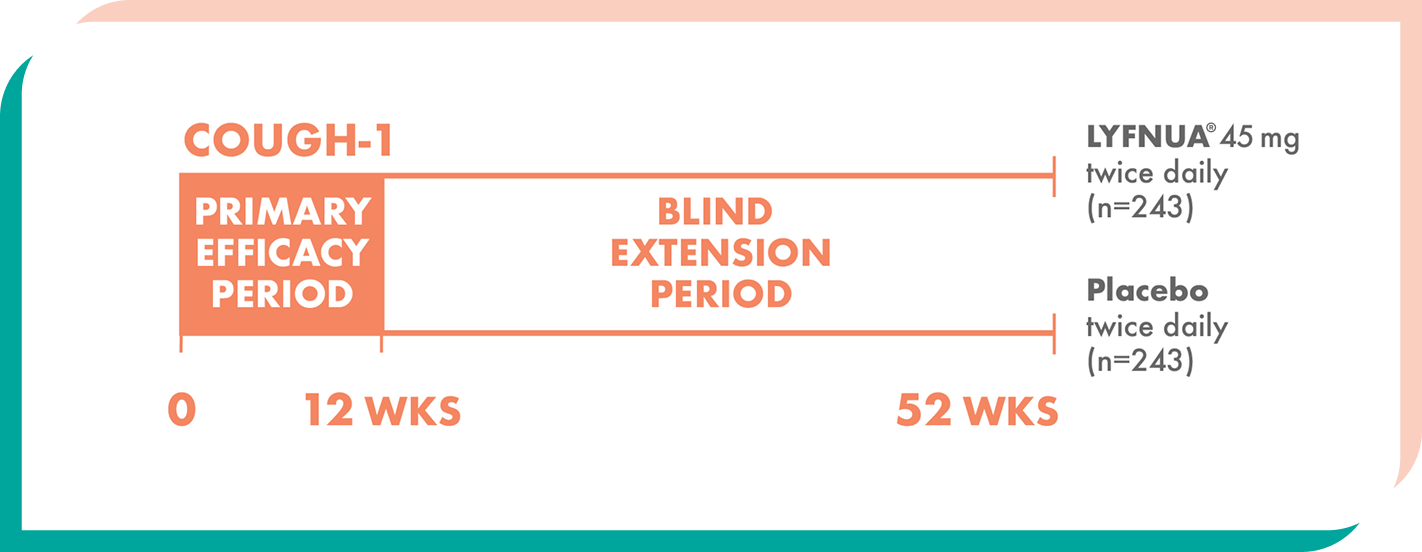
LYFNUA® was evaluated in two phase 3 clinical trials, including extensions up to Week 521

PRIMARY OBJECTIVE:
Reduction in 24-hour cough frequency relative to placebo1

SECONDARY OBJECTIVES:
Reduction in awake cough frequency and cough-specific quality of life1

COUGH-1 and COUGH-2:
Two 52-week, multicenter, randomized, double-blind, placebo-controlled studies of adults (N=2044) with either refractory or unexplained chronic cough1,2
Patients were randomized to twice-daily doses of LYFNUA® 45 mg, 15 mg (not shown), or placebo1
Safety was evaluated in 1369 patients from COUGH-1 and COUGH-2 treated with LYFNUA® (15 mg or 45 mg twice daily) over 52 weeks.1
Patients with a range of age, cough duration, and most common comorbid conditions were enrolled in both trials1,3
Select baseline demographics1,3:

Percentage of female patients: 75%

Mean age: 58 years old (range 19 to 89)

Mean duration of chronic cough: 11 years

Percentage of paatients diagnosed with refractory chronic cough: 61.5%

Percentage of patients diagnosed with unexplained chronic cough: 38.5%

Most common comorbid conditions: asthma, GERD, and allergic rhinitis2
GERD, gastroesophageal reflux disease; refractory chronic cough, cough associated with a comorbid condition that persists despite adequate treatment of the comorbid condition; unexplained chronic cough, cough not associated with a comorbid condition despite a thorough evaluation.1
COUGH-2: Reduction in 24-hour cough frequency at 24 weeks relative to placebo was -13.29%* (95% Cl: -24.74, -0.10; p=0.048)
Clinical Study Design
EXPLORE THE PHASE 3 CLINICAL TRIALS
A landmark clinical program
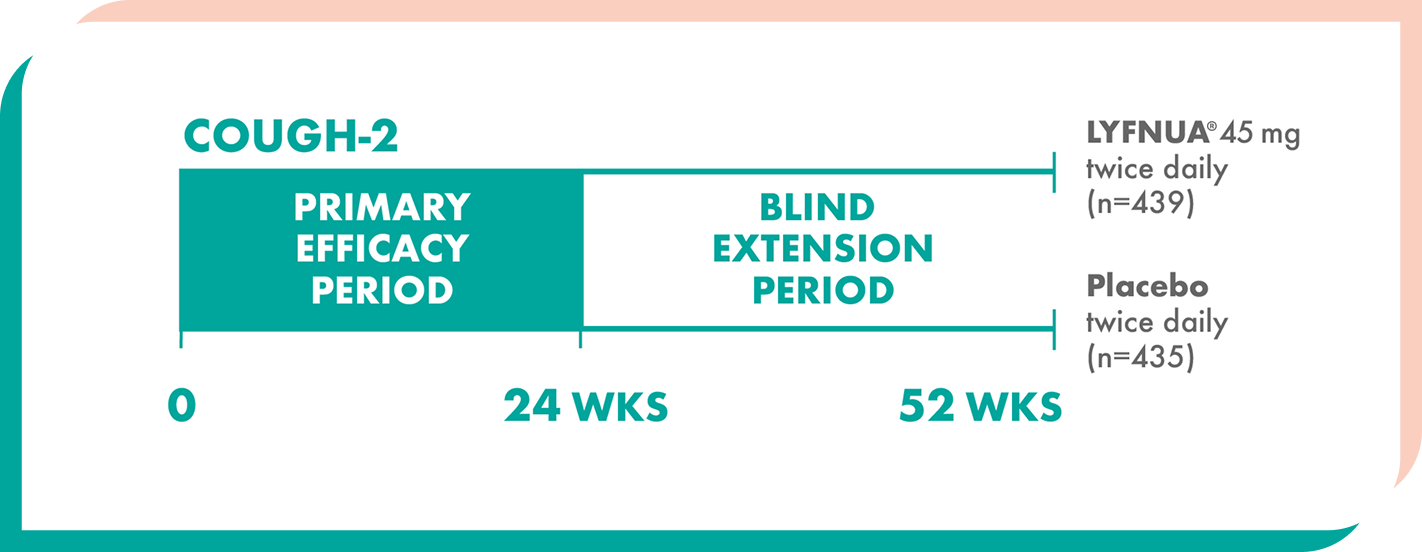
LYFNUA® was evaluated in two phase 3 clinical trials, including extensions up to Week 521

PRIMARY OBJECTIVE:
Reduction in 24-hour cough frequency relative to placebo1

SECONDARY OBJECTIVES:
Reduction in awake cough frequency and cough-specific quality of life1

COUGH-1 and COUGH-2:
Two 52-week, multicenter, randomized, double-blind, placebo-controlled studies of adults (N=2044) with either refractory or unexplained chronic cough1,2
Patients were randomized to twice-daily doses of LYFNUA® 45 mg, 15 mg (not shown), or placebo1


Safety was evaluated in 1369 patients from COUGH-1 and COUGH-2 treated with LYFNUA® (15 mg or 45 mg twice daily) over 52 weeks.1
Patients with a range of age, cough duration, and most common comorbid conditions were enrolled in both trials1,3
Select baseline demographics1,3:

Percentage of female patients: 75%

Mean age: 58 years old (range 19 to 89)

Mean duration of chronic cough: 11 years

Percentage of patients diagnosed with refractory chronic cough: 61.5%

Percentage of patients diagnosed with unexplained chronic cough: 38.5%

Most common comorbid conditions: asthma, GERD, and allergic rhinitis2
GERD, gastroesophageal reflux disease; refractory chronic cough, cough associated with a comorbid condition that persists despite adequate treatment of the comorbid condition; unexplained chronic cough, cough not associated with a comorbid condition despite a thorough evaluation.1

*The Leicester Cough Questionnaire is a validated, multidimensional, patient-reported, health-related quality of life questionnaire commonly used in clinical studies assessing cough. It evaluates physical, social, and psychological components of cough-specific quality of life.3 A ≥1.3 point increase from baseline in LCQ total score was defined as clinically meaningful.
Clinical Study Design
EXPLORE THE PHASE 3 CLINICAL TRIALS
A landmark clinical program
LYFNUA® was evaluated in two phase 3 clinical trials, including extensions up to Week 521

PRIMARY OBJECTIVE:
Reduction in 24-hour cough frequency relative to placebo1

SECONDARY OBJECTIVES:
Reduction in awake cough frequency and cough-specific quality of life1

COUGH-1 and COUGH-2:
Two 52-week, multicenter, randomized, double-blind, placebo-controlled studies of adults (N=2044) with either refractory or unexplained chronic cough1,2
Patients were randomized to twice-daily doses of LYFNUA® 45 mg, 15 mg (not shown), or placebo1


Safety was evaluated in 1369 patients from COUGH-1 and COUGH-2 treated with LYFNUA® (15 mg or 45 mg twice daily) over 52 weeks.1
Patients with a range of age, cough duration, and most common comorbid conditions were enrolled in both trials1,3
Select baseline demographics1,3:

Percentage of female patients: 75%

Mean age: 58 years old (range 19 to 89)

Mean duration of chronic cough: 11 years

Percentage of patients diagnosed with refractory chronic cough: 61.5%

Percentage of patients diagnosed with unexplained chronic cough: 38.5%

Most common comorbid conditions: asthma, GERD, and allergic rhinitis2
GERD, gastroesophageal reflux disease; refractory chronic cough, cough associated with a comorbid condition that persists despite adequate treatment of the comorbid condition; unexplained chronic cough, cough not associated with a comorbid condition despite a thorough evaluation.1
CLICK THE FOLLOWING TO LEARN MORE
INDICATION
LYFNUA® is indicated in adults for the treatment of refractory or unexplained chronic cough (RCC).1
References:
- LYFNUA®, Summary of product characteristics, 09/2023, section 4.1, 5.1 and 4.8
- McGarvey LP, Birring SS, Morice AH et al., COUGH-1 and COUGH-2 Investigators. Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet. 2022 Mar 5;399(10328):909-923.
- Muccino DR, Morice AH, Birring SS et al., Design and rationale of two phase 3 randomised controlled trials (COUGH-1 and COUGH-2) of gefapixant, a P2X3 receptor antagonist, in refractory or unexplained chronic cough. ERJ Open Res. 2020 Nov 2;6(4):00284-2020.
▼ LYFNUA® (gefapiksant) 45 mg filmdrasjerte tabletter
Terapeutisk indikasjon: Lyfnua er indisert til voksne til behandling av refraktær eller uforklarlig kronisk hoste.
Dosering og administrasjonsmetode: Anbefalt dose av Lyfnua er én 45 mg tablett tatt oralt to ganger daglig med eller uten mat. Pasienter med alvorlig nyresvikt (eGFR < 30 mL/minutt/1,73 m²) som ikke krever dialyse, bør få en redusert dose på én 45 mg tablett som tas én gang daglig.
Pakker og priser (MRP): 56 filmdrasjerte tabletter (blisterpakke), 45 mg: kr 1445,90. Reseptgruppe: C.
Utvalgt sikkerhetsinformasjon:
Spesielle advarsler og forholdsregler for bruk: For pasienter med OSA (obstruktiv søvnapné), bør passende behandling for OSA vurderes før behandling med Lyfnua igangsettes. Lyfnua bør brukes med forsiktighet hos pasienter med kjent overfølsomhet for sulfonamider. Behandling med gefapiksant skal vurderes og tilpasses hos pasienter som utvikler en akutt infeksjon i nedre luftveier.
Fertilitets-, graviditets- og ammeinformasjon: Bruk av Lyfnua bør unngås under graviditet, hos kvinner med reproduksjonsmuligheter som ikke bruker prevensjon, og for ammende kvinner.
Bivirkninger: De vanligste bivirkningene med Lyfnua (som kan påvirke mer enn 1 av 10 personer) inkluderer smakforstyrrelser: dysgeusi (endringer i smakssansen, 41%), ageusi (tap av smakssans, 15%) og hypogeusi (redusert smakssans, 11%).
Konsulter LYFNUA SmPC 09/2023 før forskrivning eller bruk for fullstendig informasjon om dosering, kontraindikasjoner, advarsler og forholdsregler, interaksjoner og bivirkninger.
MSD (Norge) AS, Postboks 1S79 Vika, 0118 Oslo, tlf. 32 20 73 00 faks 32 20 73 10.
Copyright © 2025 Merck & Co., lnc., Rahway, NJ, USA og dets datterselskaper. Alle rettigheter forbeholdt.
NO-NON-00562